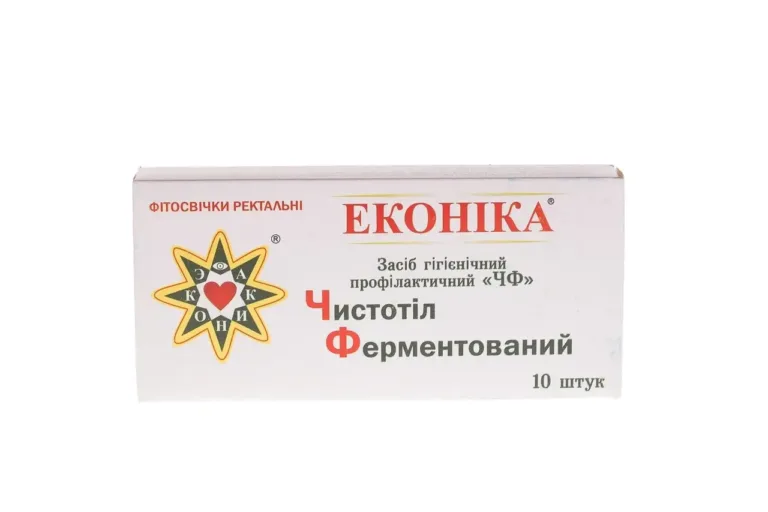
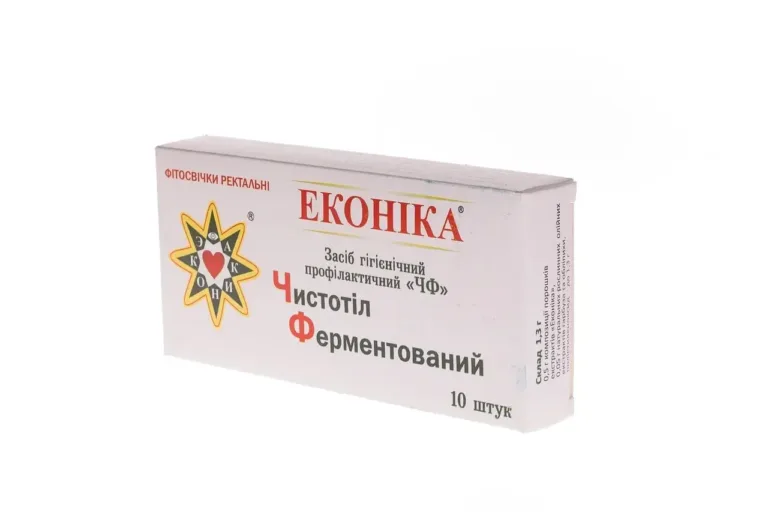
Phytosuppositories “Fermented Celandine” are a natural preparation that undergoes special processing.
- Celandine juice is fermented in an environment of colostrum and goat milk serum and medicinal plants using a special technology.
- Fermentation takes place within 7 calendar months, after which celandine acquires new unique properties.
- The drug Fermented Celandine is absolutely non-toxic and non-poisonous. Phytosuppositories are non-toxic and have anti-inflammatory, antitumor, bactericidal, antifungal, anesthetic and antispasmodic properties.
Composition
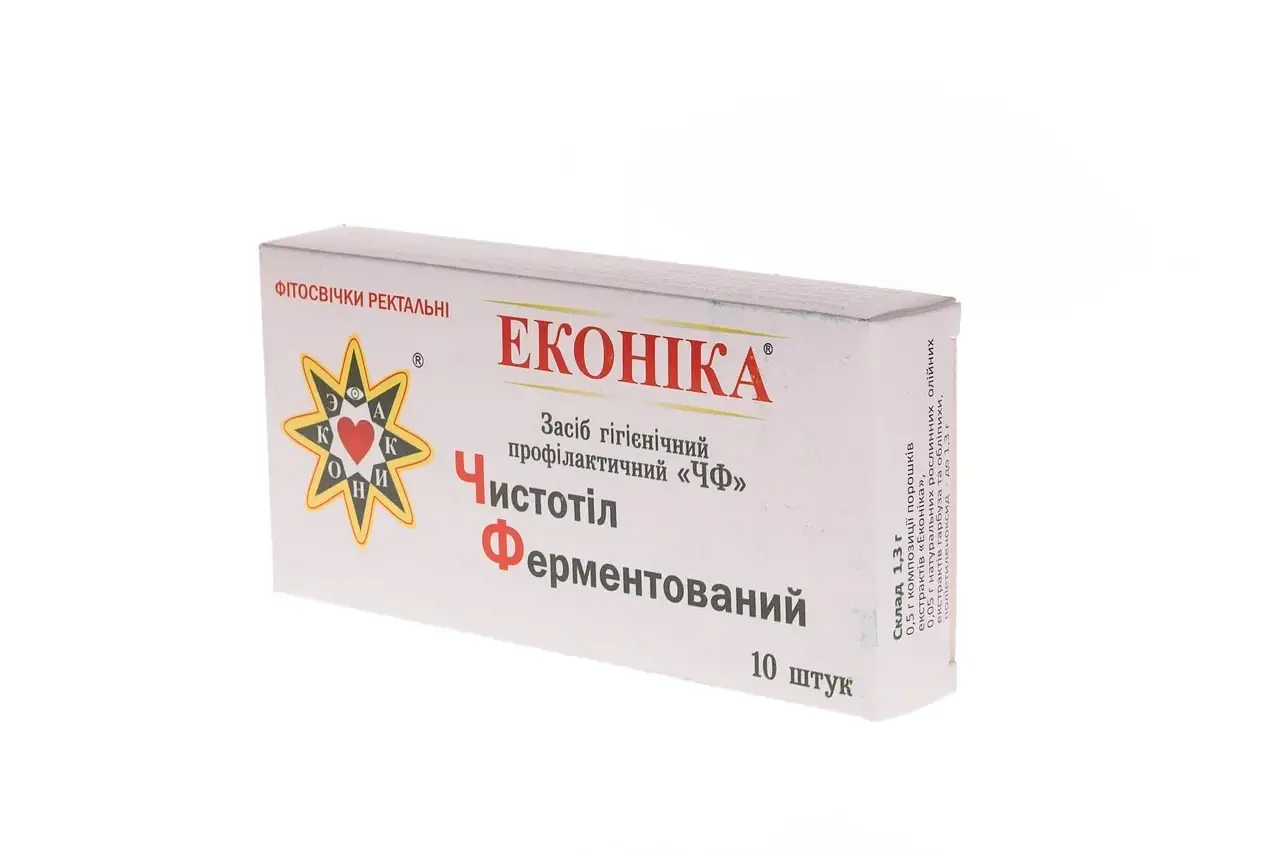
- 0.5 g composition of powders of “Econika” extract;
- 0.05 g of natural oil extract of pumpkin and sea buckthorn;
- polyethylene oxide to 1.3g
Application
- Phytosuppositories “Fermented celandine” are used for the prevention and treatment of chronic diseases at all stages of the course.
- Can be used in monovariant and complex prophylaxis.
- In combination with radio- and chemotherapy, the drug reduces side effects.
- Phytosuppositories regulate metabolic processes, normalize digestion, have a general strengthening effect, have an analgesic effect, and have a lytic effect on tumor cells.
- When used, patients experience subjective improvement or a feeling of being healthy;
- objective indicators of blood tests, tumor markers, computed tomography results, etc. are normalized;
- the body’s drainage functions are enhanced (binding and excretion toxins);
- symptoms of intoxication disappear;
- the state of the psyche improves (improved sleep, reduced depression, even calm mood, reduced irritability, vulnerability, etc.);
- antimetastatic and anti-relapse effect
Indications for use
- immunodeficiency states;
- infectious purulent and septic processes;
- benign tumors;
- malignant neoplasms;
- melanomas;
- chronic viral hepatitis;
- tuberculosis;
- lymphogranulomatosis;
- leukemia;
- in surgical interventions (to accelerate healing wounds);
- resorption of adhesions and scars;
- Bechterew’s disease;
- with overwork,
- huge physical exertion for quick recovery.
Contraindications: no contraindications have been identified.
Method of application and dosage: as prescribed by a doctor rectally, vaginally, 1 suppository 1-2 times a day.
Course of treatment or prevention: 1 month.
It is recommended to take according to the scheme: 10 days of intake, 5 days of break and again, 10 days of intake, 5 days of break.
Overdose: There were no cases of overdose.
Form of release: in one cardboard package 10 suppositories in plastic cells.
Weight of suppository: 1.3 g
Storage conditions: at a temperature of 4ºС to 18ºС
Expiration date: 2 years from the date of manufacture.
Not a medicinal product
TU U 24.4-1945600658-004: 2005
GMO-free
Manufacturer: PP Borodatov A.I
You must be logged in to post a review.



















Reviews
There are no reviews yet.